Investigating the Ways of Nature: An In-Class Experiment Professor, Program of Liberal Studies and Graduate Program in History and Philosophy of Science University of Notre Dame Notre Dame, IN 46556 USA It has been my experience that students tend to make fewer of the discoveries than one might think from reading the paper, but this has essentially no bearing on what they derive from the experiment. What seems to be the most important lesson that they learn is that the ways of nature are rather more difficulty to decipher than they had thought. Correspondingly, they are less ready to criticize earlier scientists for failing to arrive at modern theories. It may be useful to note that if persons wishing to use the experiment have difficulty obtaining 1943 pennies, I can to a limited extent help. Write me at the above address. Because no diagram appeared in the original paper, I have prepared a diagram of the can for this presentation. It follows the original paper. BLACK BOXES AND WHITE CANS: An Effective Experiment for Use in College Science Courses -- and Elsewhere: In the April 1961 issue of JGE: The Journal of General Education, Professors A. J. Bernatowicz and E. A. Kay described a laboratory experiment they used in science courses. Stating that they had successfully used the experiment with both undergraduate and graduate students, they promised large dividends to readers who would go and do likewise. This reader followed their suggestion and, after using a modified form of their experiment for six years, reports that their experiment works well not only in undergraduate and graduate courses, but also at student and faculty parties -- a dividend they did not mention. I want to remind current readers of the gold buried in that back issue and to describe a modified form of the experiment which seems to increase its educational benefits. The original form of the experiment, called the black box experiment, was simple. The authors of the 1961 paper presented their students with a cigar box, painted black, containing a variety of dime-store items, some fastened, some loose. The lid is nailed shut, and a few small holes are drilled through the walls. For contents they used some wood strips fastened as baffles, a magnet, some rolling objects, and wads of such materials as sheet agar and plastic sponge whose texture and rustling are much like those of other materials. They recommended that for twenty students divided into five teams, five such boxes be used. The students were asked to investigate the box and its contents, mak-ing use of various pieces of equipment set out on laboratory tables. Upon the completion of their hour's investigation, the students reported on results and discussed their methods of inves-tigation and of proof. The authors of the original paper pointed out many benefits derived from the use of this experiment; some of these benefits as well as others not noted before will be listed after the modified form of the experiment is described. It too is simple. The (pipe-smoking) author of the present paper recommends a device which differs in a number of ways from the black cigar box; for example, his black box is a white, one-pound, pipe-tobacco can. More importantly, his procedure is more open, and his white can reveals more secrets to those stu-dents who are cunning enough to seek them out. Let us try the experiment. You are presented with one hour and one white can sealed shut by tape. From a small hole in the side of the can, a loop of string protrudes. You are told that the rattle in the can comes from a penny suspended in the can and that six or more definite and interesting discoveries await the in-genious experimenter. You ask whether any instruments are available for use in the investigation and are told, "Yes -- but only to those who explicitly ask for them." One hour later, if you are rather more clever than any person I have encountered, you may hand in a report that runs as follows:
Though no student has reported all of the above, every student has been able to say part of it -- and invariably has said it with the excitement and delight that are the rewards of discovery. After the teams announce their results (and after back-slapping has given way to head-slapping), the fun is over, but the most important part of the procedure begins. The students (either in discussion or in individual papers) analyze their methods and results (or lack thereof). From this they learn about a number of aspects of scientific methodology; some of these are listed below. 1. The difference between statements of observation and statements of inference. The student who reports that there is a balloon or a cap in the can has failed to appreciate the difference between an observation and an inference. The discussion helps him to see this difference and to understand how natural it was for Herschel to announce that he had discovered a new comet, when he had in fact discovered the planet Uranus. 2. The difference between a raw observation and a refined observation. A thoughtful student commented in the analysis of the experiment: "One example of how close we came to one of the phenomena came out after the experiment was over. A member of the group commented on the peculiar smell of the can. Another, from a distance, suggested that it was the smell of the tobacco that had been in it. Still a third said that he had smelled something peculiar, but that he had not connected it with the can since the person who was holding it seemed to be chewing something." Another student added that he had smelled the Vicks, but "figured someone had a cold." Students learned that raw observations are of almost no value and they henceforth were less mystified by the fact that countless generations of doctors failed to suspect a relation between their unclean hands and the occurrence of infection in their patients. 3. The misleading effect of a wrong or unnecessarily narrow hypothesis. The first student quoted above went on to comment: "we couldn't possibly have noticed the smell, since we had become convinced that all discoveries were related to the penny and its suspension." This group was readier to understand how Agassi and other scientists could accept Darwin's observations, but reject his theory, since they had set those observations into a different Gestalt. 4. Absurd hypotheses sometimes suggest important discoveries.A winning team explained their success in the following way: from knowing the penny (money) was in the can, they hypothesized that the can contained something for all of man's needs. Medicine, for instance -- hence they discovered the menthol-odor-producer. Man must breathe; hence air. Man must eat and drink; hence the water-colorer and water-sweetener. Their hypothesis was clearly absurd, but it was also extremely suggestive. They thus discovered a truth known to Kepler scholars: a man who has wild ideas but also a respect for observation sometimes attains spectacular results. 5. "Obvious" is a term to be used with caution. Nearly every group asks to use a magnet during the experiment and they diligently apply the magnet to the outside of the can, but few groups get the "obvious" idea of investigating whether a magnet may be inside the can. To us it is obvious that children resemble both parents; to Aristotle and the Greeks it was obvious that this statement is false. 6. The nature of accidental discovery. One student re-vealed how he had discovered the magnet: "I wasn't getting any ideas and was absent mindedly tapping the top of the can with my pen when..." This led to fruitful discussion of the nature of accidental discoveries and of Pasteur's famous statement that "chance favors the prepared mind." 7. The wrong experiment sometimes gives the right result. One group decided to investigate the flotation properties of the can, but were for a time bothered by water leaking into the can ... and Fleming was for a time bothered by the mold that was spoiling his cultures. 8. An experimental test may be right, but its intensity wrong. Only about half of the students who heat the can discover the pop-producer. Their experimental method is qualitatively correct, but they fail to apply the heat either with sufficient intensity or for an adequate length of time. Those who fail can sympathize with Faraday; those who succeed can rejoice with Zeeman. 9. A scientific discovery usually consists of two parts: the discovery of the method for solving the problem and the discov-ery of the solution of the problem. Discussion frequently turns to the question of when a certain discovery was made. Student A suggests putting water into the can, student B puts water into the can and discovers the water-reddener. When was the discovery made? Students who have faced this question are ready to discuss the question of whether Lalande or Adams and Lever-rier or Galle discovered Neptune and to discuss what the term "discovery" means. 10. The discovery of the answer to a question is usually primarily the discovery of another question. The students invariably ask to see the contents of the can. The authors of the earlier paper, after making the same comment, stated that "here the most important lesson" can be learned. In their response, they "emphasize that it is futile to hope for final answers in science." Perhaps I am more of a realist. In any case, I prefer to open one of the cans (a special one) -- and slowly to lift out a two-ounce size white tobacco can! Enthusiasm for this experiment, which has been evident in the students I have observed, leads me to give a strong second to the earlier suggestion of Professors Bernatowicz and Kay. The experiment not only gives students a far richer understanding of ideas relating to scientific verification and discovery, but also, and more importantly, gives them the same feeling of excitement that led to that statement, first made by Archimedes, but re-peated so frequently in the annals of the history of science -- Eureka! 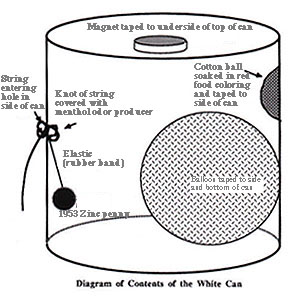
© 1998 by the History of Science Society, All Rights Reserved |